Noble gases are known to be stable and unreactive. The common ionic charge of boron is 3.
Atomic Structure The Atom Boron Losing Electrons
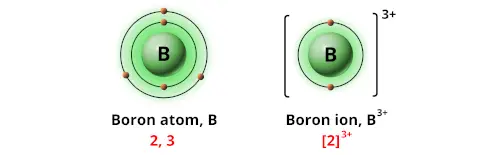
What is the charge on boron when it becomes an ion.
. First find the number of electrons an atom of Boron usually has. The imbalance of protons and electrons now creates a charge of 3 on the atom. This means it has 5 protons.
The impure amorphous product a brownish black powder was the only form of boron known for more than a centuryPure crystalline boron may be prepared with. When an electron is added to a neutral atom energy is released. Boron status is not routinely measured in clinical practice.
Aluminium is a highly electropositive element. This is due to the small size of boron which makes the sum of its first three ionization enthalpies very high. So on a periodic table Boron has an atomic number of 5.
Most studies suggest that urinary boron levels correlate with boron intakes 241617. Helium is the first element in the noble gas series. Boron was first isolated 1808 by French chemists Joseph-Louis Gay-Lussac and Louis-Jacques Thenard and independently by British chemist Sir Humphry Davy by heating boron oxide B 2 O 3 with potassium metal.
In pure boron as well as boron rich solids like boron carbide and B6O the bonds of the atoms that make up the chains ie. To use electron affinities properly it is essential to keep track of sign. You can see that it has 3 more positive charges than it usually has.
Now look at the charge. 93 rows Charge of Helium ion. Boron is excreted mainly in the urine and small amounts are excreted in the feces sweat breath and bile 910.
Boron B is a chemical element with an atomic number 5 that belongs in the Period 2 and Group 13 in the periodic tableIt is a low-abundant metalloid that is a poor electrical conductor at room temperature. Boron is an element. So usually Boron B has 5 electrons.
Boron-11 is the stable isotope of boron with relative atomic mass 11009306 801 atom percent natural abundance and nuclear spin 32. Atoms with a charge are known as IONS. What is the common ionic charge of boron.
B e B H Affinity 267 kJmol. Boron has been consumed for menstrual cramps and boric acid has been used vaginally for yeast infections but evidence is limited. The bonds linking the icosahedra.
While moving from B to Al the sum of the first three enthalpies drastically decreases. In this video we will write the electron configuration for B 3 the Boron ion. More precisely atoms with a positive charge are known as cations while atoms with a negative charge are known as anions.
It is also found for applications including ceramics. Boron ion is represented as B singly charged positive. For Boron it would readily want to lose 3 electrons so as to mimic a Helium He atom.
This results in the formation of Al 3 ions. When Boron loses 3 electrons a net charge of 3 is left on the atom of Boron. Natural boron exists in 2 stable isotopes however pure boron is hard to prepare due to contamination by different elements.
C-B-C C-B-B B-B O-B-O etc bonded to the B12 B11C or B10C2 icosahedra are stronger than the bonds that make up the icosahedra. Boron has three electrons in the outer energy level. Charge of Lithium ion.
Boron generally forms covalent bonds rather than 3 ions. An atom of Boron in the gas phase for example gives off energy when it gains an electron to form an ion of Boron. Having 5 protons it also has 5 electrons.
ChEBI A trace element with the atomic symbol B atomic number 5 and atomic weight 10806. Well also look at why Boron forms a 3 ion and how the electron configuratio.

Boron Properties Uses Facts Britannica
0 Comments